Infection biology /Bacterial pathogenesis
The genus Acinetobacter has been invaluable to study unusual metabolic capabilities for microbes such as growth on aromatic compounds and is well-known to many microbiologists (Young et al., 2005). One representative, the soil bacterium A. baylyi, and its tremendous capability for DNA uptake has been an eye-opener for students since decades, introducing students to the concept of natural transformation and has been subject of intensive studies in our group (Averhoff and Graf, 2008). However, there are also representatives within this genus, such as Acinetobacter baumannii, which are emerging pathogens in clinical environments and their multidrug-resistances have become a global challenge (Göttig et al., 2010; Peleg et al., 2012). Our prime interest focusses on the understanding adaptation of pathogenic bacteria to the human host and the clinical environment.
Acinetobacter baumanni – understanding and fighting a new emerging pathogen
In recent years it became more and more evident that A. baumannii can cause life-threatening infections - as recently evidenced by the infections of soldiers in military hospitals after returning from the Iraq war (“Iraqibacter”) and a growing number of epidemic outbreaks, e.g. in intensive car units (ICUs) all over the world. A. baumannii is now among the top 10 pathogens. However, alarmingly little is known about the molecular basis of host adaptation. Phosphatidylcholine (PC) is one of the most abundant phospholipids in human cell membranes and makes it a good candidate as carbon and energy source. Initial degradation of PC is mediated by phospholipases liberating glycerol, fatty acids and choline which might act as carbon and energy source thereby fostering the metabolic adaptation to the human host. A. baumannii encodes a whole set of phospholipases which are currently subject of molecular and biochemical analyses. Transcriptional and immunological analyses will identify conditions under which the encoding genes are expressed. We have established a novel molecular toolbox based on a markerless mutagenesis system. Mutant studies using eukaryotic host systems will be a prime focus of our future studies and unravel the role of the phospholipases in pathogenicity and persistence in the human host. This understanding will, in a long run, help to either disarm A. baumannii, by taking preventive measures or to identify new targets for (antibiotic) treatment. These pressing questions are addressed in a collaborative effort in the network of a research unit which has been installed by the German Science Foundation last year (http://www.bio.uni-frankfurt.de/51172482).
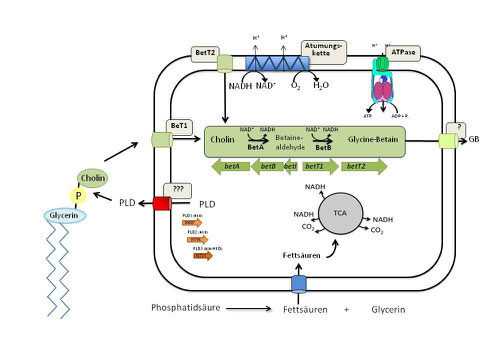
Model of Phosphoatidylcholine conversion by A. baumannii.
References
Peleg, A.Y., de Breij, A., Adams, M.D., Cerqueira, G.M., Mocali, S., Galardini, M., Nibbering, P.H., Earl, A.M., Ward, D.V., Paterson, D.L., Seifert, H., and Dijkshoorn, L. (2012) The success of Acinetobacter species: genetic, metabolic and virulence attributes. PLoS One 7: e46984.
Göttig, S., Pfeifer, Y., Wichelhaus, T.A., Zacharowski, K., Bingold, T., Averhoff, B., Brandt, C., and Kempf, V.A. (2010) Global spread of New Dehli metallo-ß-lactamase 1. Lancet Infect Dis 10: 828-829.
Averhoff, B., and Graf, I. (2008) The natural transformation system of Acinetobacter baylyi ADP1: a unique DNA transport machinery, p. 119-140 In U. Gerischer (ed.) Acinetobacter Molecular Biology, Caister Academic Press: Norfolk, UK.
Young, D.M., Parke, D., and Ornston, L.N. (2005) Opportunities for genetic investigation afforded by Acinetobacter baylyi, a nutritionally versatile bacterial species that is highly competent for natural transformation. Annu Rev Microbiol 59: 519-555.

